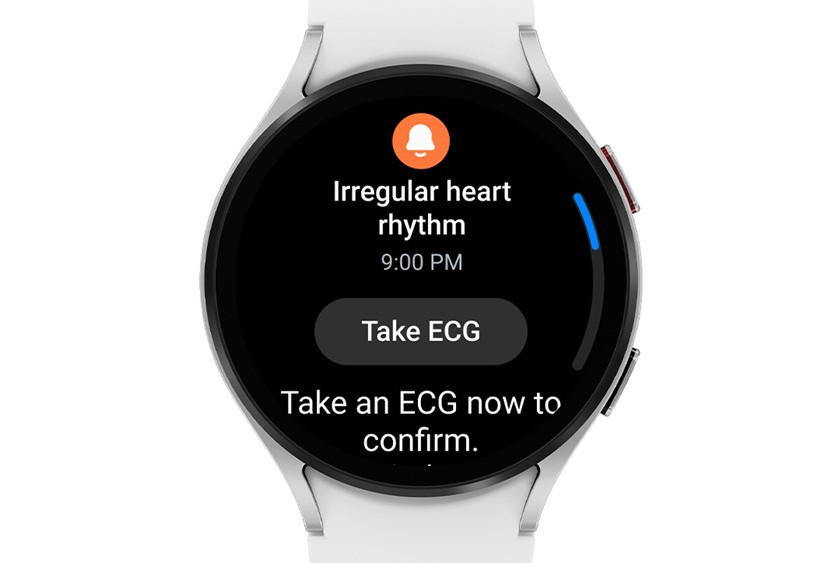
Samsung has announced that it is brining Irregular Heart Rhythm Notification (IHRN) feature to Galaxy Watch to 13 markets starting this summer. This comes after the company received FDA approval for the feature last month.
The Irregular Heart Rhythm Notification (IHRN) feature got Korean Ministry of Food and Drug Safety (MFDS) approval last week, said the company.
It will also be introduced in Argentina, Azerbaijan, Costa Rica, Dominican Republic, Ecuador, Georgia, Guatemala, Hong Kong, Indonesia, Panama, UAE, as well as Korea and the U.S., taking the total to 13 markets.

Combined with the Blood Pressure and Electrocardiogram (ECG) monitoring, the IHRN feature checks for irregular heart rhythms in the background and warns the user of potential AFib activity.
Together with the existing Blood Pressure and Heart Rate monitoring, users can gain even deeper insights into their cardiovascular health, said the company.
Availability
The Irregular Heart Rhythm Notification feature will first be available on the upcoming Galaxy Watch devices later this year in these markets, as part of the new One UI 5 Watch and later expanded to previous editions.
Regardin this, Hon Pak, Vice President and Head of Digital Health Team, MX Business at Samsung Electronics, said:
Cardiovascular disease remains one of the main causes of death around the world, and we’re committed to empowering our users to stay informed about their heart health by providing monitoring tools, including blood pressure measurement and irregular heart rhythm notification. We’re constantly exploring new ways to help Galaxy Watch users obtain deeper insights on their health and wellness effortlessly, day and night.
